Assessment of patients affected by rheumatoid arthritis eligible for biotechnological agents and evaluation of their healthcare resource utilization and related costs
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
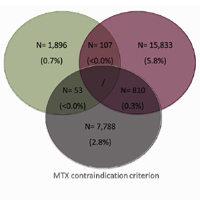
Objective. To provide estimates of patients with rheumatoid arthritis (RA) eligible for biotechnological therapy and to evaluate their healthcare costs. Method. An observational analysis was performed based on data-linkage between administrative databases of selected Italian Regional/Local healthcare departments. Data were then re-proportioned to the Italian population. Patients with RA diagnosis defined by discharge diagnosis and/or exemption code during 01/01/2013- 31/12/2017 were included. The criteria applied to evaluate the elegibility for biotechnological therapy were: 1) methotrexate (MTX)-treatment failure ≥6 months and start of a different conventional-synthetic diseasemodifying antirheumatic drugs (csDMARD); 2) corticosteroid ≥6 months with dosage ≥7.5 mg/die; 3) MTX-contraindication (therapy or hospitalization for renal damage/interstizial lung disease/hepatic failure). Mean annual costs per patient included drugs, hospitalizations, outpatient services. Results. Data re-proportioned to the Italian population estimated 318,328 RA patients: 43,361 with, 274,967 without biotechnological agents. Among the latter, 26,487(9.6%) patients met ≥1 criteria applied for eligibility: 1,896 had MTX-treatment failure and started another csDMARD; 15,833 received corticosteroid ≥7.5 mg/die; 7,788 had MTX-contraindication. Regarding patients fulfilling two criteria, 107 had MTX-treatment failure followed by another csDMARDs and corticosteroid ≥7.5 mg/die, 53 were treated with another csDMARDs after MTX-treatment failure and also presented MTX-contraindication, 810 had corticosteroid ≥7.5 mg/die and MTX-contraindication. Mean total annual costs for patients estimated eligible for biotechnological therapy was € 3,132, of which € 177 related to drugs indicated for RA and € 2,955 related to other direct costs. Conclusions. According to our estimates, around 10% RA patients not currently treated with biotechnological agents are eligible for such therapies, highlighting a trend of under-use in clinical practice for RA management.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.










